What We Create
Graphite Anode Material
In lithium-ion batteries, graphite is used as an anode material due to its ability to intercalate lithium ions between its layers during charging. This ensures high electrical conductivity, stability over multiple charge-discharge cycles, and sufficient capacity for energy storage. Despite the search for more efficient alternatives, graphite remains the key anode material because of its availability and well-established application technology.
Why graphite matters:
-
Lithium Ion Storage
Graphite has a layered structure that allows lithium ions (Li⁺) to intercalate between layers during charging — like “absorbing” lithium without destroying the structure.
-
Stability and Longevity
- Chemically inert within the battery’s voltage range
- Minimal expansion/contraction (~10%)
-
Conductivity
Graphite conducts electrons efficiently — essential for current flow in the external circuit.
-
Safety
Unlike pure lithium, graphite prevents dendrite formation (sharp lithium spikes) that could pierce the separator and cause short circuits.
-
Affordability and Availability
Graphite is a relatively low-cost material compared to alternatives like silicon.
What It’s Made Of
Graphite
Graphite is an allotropic form of carbon with a hexagonal layered crystalline structure. Each layer consists of hexagonal cells with strong covalent bonds between atoms, while weak van der Waals forces between layers give graphite its softness, conductivity, and ability to delaminate.
Graphite’s unique combination of properties — electrical conductivity, thermal resistance, chemical inertness, and delamination — makes it a truly versatile material with no full substitute in most applications.
Its properties make it indispensable across industries:
- Metallurgy and Manufacturing
- Electrical Engineering and Energy
- Mechanical Engineering
- Chemical Industry
- Aerospace
Withstands temperatures up to 3000°C. Used in crucibles, casting molds, and electrodes for arc furnaces.
Conducts electricity due to mobile electrons between layers. Used in lithium-ion battery anodes, current collectors, and motor components.
Acts as a dry lubricant in high-temperature mechanisms (engines, bearings), thanks to layer slippage.
Resistant to aggressive media. Used in seals, heat exchangers, and reactors for acids and alkalis.
Lightweight yet strong — ideal for parts in rockets, satellites, and aircraft.
What It’s For
Lithium-Ion Battery
Lithium-ion batteries are the technological standard of the 21st century — combining efficiency, versatility, and sustainability. Their development drives the transition to clean energy and electric mobility.
Advantages:
-
High Energy Density
Stores more energy per unit of weight — compact and lightweight.
-
Low Self-Discharge
Loses just 1–5% charge per month, retaining energy longer.
-
Long Life Cycle
Withstands 500–1500 charge cycles before capacity drops by 20%.
-
Fast Charging
Tolerates high current without major structural degradation.
-
Eco-Friendliness
Free from toxic heavy metals like lead or cadmium.
Applications in modern technology:
- Portable Electronics — smartphones, laptops, tablets
- Electric Vehicles — long range (Tesla, Nissan Leaf, BYD, etc.)
- Green Energy — stores power from solar and wind sources
- Medicine — powers implants and portable medical devices
Lithium-ion batteries reduce dependence on fossil fuels and cut CO₂ emissions.
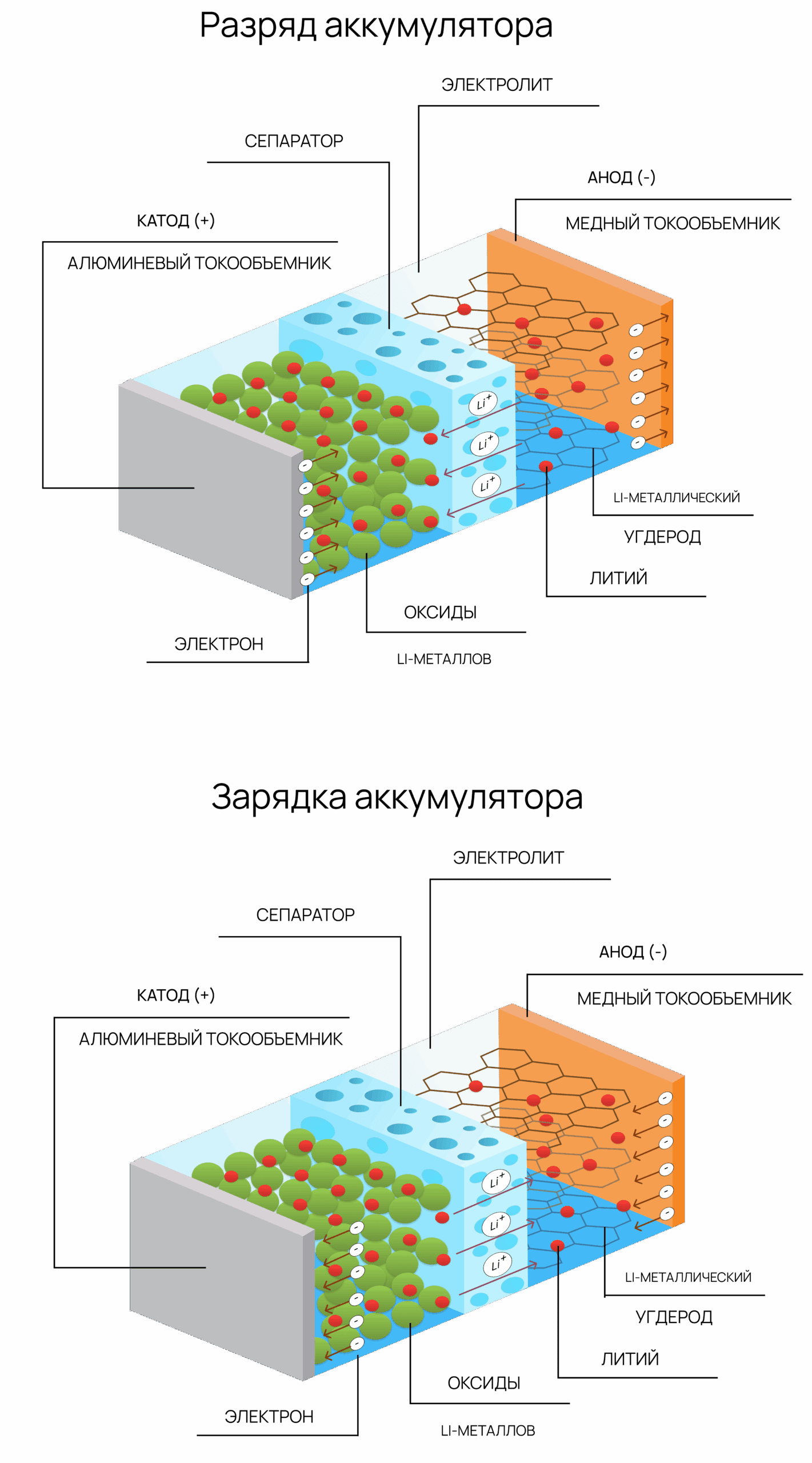
How a Li-ion battery works:
- Main components:
- Anode (usually graphite) – releases lithium ions during discharge
- Cathode (metal oxide, e.g., LiCoO₂) – absorbs lithium ions
- Electrolyte – lithium salt in organic solvent, conducts Li⁺
- Separator – isolates electrodes and prevents short circuits
- Discharge (in use):
- Li⁺ ions move from anode to cathode through electrolyte
- Cathode (metal oxide, e.g., LiCoO₂) – absorbs lithium ions
- Electrons travel through the external circuit, powering devices
- Charging:
- External current drives Li⁺ ions back to the anode
- Electrons return via the charging circuit